Health
WHO Endorses World’s First Malaria Vaccine
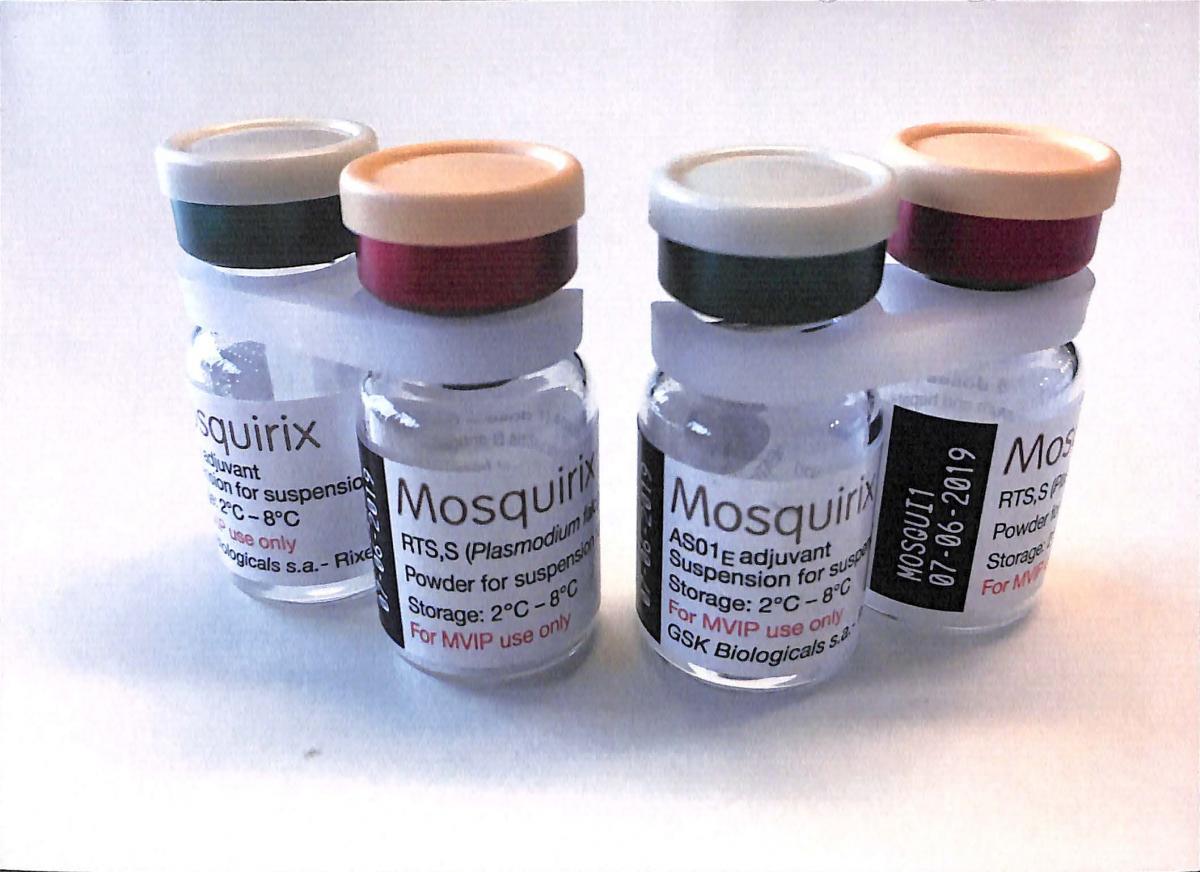
By Adedapo Adesanya
The World Health Organization (WHO) on Wednesday endorsed the first-ever vaccine for malaria after many years of waiting.
This has been regarded as a historical event as the drug would save the lives of tens of thousands of children lost to the disease in Africa each year.
Malaria is among the oldest known and deadliest of infectious diseases with data showing that it kills about half a million people each year, nearly all of them in sub-Saharan Africa — among them 260,000 children under age 5.
Called Mosquirix, the new malaria vaccine is given in three doses between ages 5 and 17 months, and a fourth dose roughly 18 months later.
Following the clinical trials, the vaccine was tried out in three countries — Kenya, Malawi and Ghana — where it was incorporated into routine immunization programs.
More than 2.3 million doses have been administered in those countries, reaching more than 800,000 children. That bumped up the percentage of children protected against malaria in some way to more than 90 per cent from less than 70 per cent
The new vaccine, made by GlaxoSmithKline, rouses a child’s immune system to thwart Plasmodium falciparum, the deadliest of five malaria pathogens and the most prevalent in Africa.
The vaccine is not just a first for malaria — it is the first developed for any parasitic disease.
Speaking on the feat, Dr Pedro Alonso, director of the WHO’s global malaria programme said, “To have a malaria vaccine that is safe, moderately effective and ready for distribution is a historical event.”
For more than 100 years, malaria research has had various vaccine candidates that never made it past clinical trials but the results with Mosquirix makes it the outlier.
This is set to replace bed nets which are considered the most widespread preventive measure as it cuts malaria deaths in children under age 5 by about 20 per cent.
Against that backdrop, the new vaccine, even with modest efficacy, is the best new development in the fight against the disease in decades, some experts said.
This week, a working group of independent experts in malaria, child health epidemiology and statistics, as well as the WHO’s vaccine advisory group, met to review data from the pilot programmes and make their formal recommendation to Dr Tedros Adhanom Ghebreyesus, the Director-General of the global health body.
The next step is for Gavi, the global vaccine alliance, to determine that the vaccine is a worthwhile investment. If the organization’s board approves the vaccine, it will purchase the vaccine for countries that request it, a process that is expected to take at least a year.
Health
SpecSMART Eye Clinic Takes Affordable, Quality Care to Ikeja, Environs

By Modupe Gbadeyanka
The dream of residents of Ikeja and its environs enjoying affordable and quality care has now become a reality as a result of the opening of a new branch of SpecSMART Eye Clinic in the Opebi area of the capital of Lagos State.
SpecSMART Eye Clinic, a leading provider of optometry services in Lagos, commenced operations in Nigeria’s commercial capital in 2022.
Since then, it has been offering top-notch eye care to residents of the metropolis, especially those living on the Island. It has built a strong reputation for delivering high-quality primary eye care and optical products.
However, to extend its services to Lagosians living on the Mainland, it has now opened a new branch in Ikeja, reinforcing its commitment to providing accessible, affordable eye care to a wider community.
Business Post gathered that the clinic’s state-of-the-art services are supported by a team of skilled optometrists and opticians, utilizing cutting-edge digital equipment.
The new Ikeja location will offer a wide range of services, including Automated Eye Examinations using advanced digital equipment for precise diagnosis and personalized care.
In addition, clients will enjoy on-the-spot lens glazing for single vision, bifocals, and varifocals, with additional lens coatings, with services to be rendered seven days a week from 9 am to 9 pm on Mondays to Saturdays, and on Sundays and public holidays from 10 am 7 pm.
Also, the clinic has over 950 frames, ranging from affordable home brands to premium designer options, priced from N18,000, and has flexible appointment scheduling with 24-hour online booking via SpecSMART’s website.
The facility has partnerships with leading HMO providers in the country and offers glaucoma management and other essential eye health services.
According to the company, its introductory packages start from N30,000 and include consultation, frame, and single-vision lenses.
“With the opening of our Ikeja branch, we are ready to serve more individuals who need accessible, cost-effective, and reliable eye care.
“Our aim is to create a positive impact in Nigeria’s optometry sector by combining advanced technology with a patient-centred approach,” the Practice Head and Medical Director of SpecSMART, Dr Adaeze Nwoko, stated.

Health
FG Begins Vaccination Against Mpox in FCT, Six States

By Adedapo Adesanya
The Federal Ministry of Health and Social Welfare through the National Primary Health Care Development Agency (NPHCDA) has commenced the vaccination against Monkeypox, now known as Mpox.
Business Post reports that Bayelsa, Rivers, Cross River, Akwa Ibom, Enugu, Benue, and the Federal Capital Territory, were selected as pilot states for the vaccination.
An average of 631 persons are expected to be vaccinated across the seven states with two doses of the Mpox vaccine. A buffer for 50 persons will be kept at the national in case of an upsurge in other states.
NPHCDA in a statement posted on its verified X account confirmed the exercise, stressing that the vaccination will help to protect communities and safeguarding health of the people.
In a related development, according to the latest update by the Nigeria Centre for Disease Control (NCDC), there are 1,442 suspected cases of Mpox from 36 states and the Federal Capital Territory, while the number of confirmed cases of the infection was 118 from 28 states and the FCT.
“To prevent the spread of Mpox, we strongly advise the public to avoid contact with animals that may carry the virus, including sick or dead animals in affected areas, avoid handling materials that have been in contact with infected animals, limit unnecessary physical contact with individuals who are infected, practice frequent handwashing with soap and water, and ensure that animal food products are thoroughly cooked before eating.
“It’s also important to use protective clothing and gloves when handling sick animals or their tissues. Similarly, health workers are advised to follow standard safety protocols including droplet precautions when treating patients, use protective equipment including masks, gloves, and gowns, during patient care, and be vigilant for symptoms of Mpox, especially fever and rash, among other measures.”
Health
AXA Mansard Health Partners LUTH in Blood Donation Drive

By Aduragbemi Omiyale
Over 250 pints of blood have been donated by AXA Mansard Health to the Lagos University Teaching Hospital (LUTH), Idi Araba.
The blood was donated by more than 100 employees of the leading health insurance company in Nigeria through its volunteering programme tagged AXA Hearts in Action.
The initiative is part of the company’s blood donation drive aimed at contributing to positive societal and environmental impacts through employee volunteering, and expertise related financial support and in-kind donations.
According to the Chief Client Officer of AXA Mansard, Ms Rashidat Adebisi, “Through the AHIA, our employees do not just give time to great causes; we work together for a better future.”
“We share our time, knowledge and expertise as a people with a shared purpose of acting for human progress by protecting what matters through initiatives like this,” she added.
Ms Adebisi said the blood drive is a shining example of the philosophy in action, where collective contributions serve as a reminder that true impact often involves giving more than just money. It’s about putting one’s heart in action – an idea embedded in AXA’s corporate culture.
On his part, the chief executive of AXA Mansard Health Limited, Mr Tope Adeniyi, said with hospitals frequently experiencing blood shortages, events like these serve as a lifeline for patients in need.
“We are proud to contribute to the local healthcare sector and provide much-needed support to hospitals such as LUTH, ensuring that they have resources essential to saving lives,” he added.
Also commenting, the Head of Corporate Services and Public Relations at LUTH, Omolola Olubukunola Fakeye, thanked the firm for the “generous support,” which has made a “meaningful difference to our blood bank and ultimately to the lives of patients.”
“Blood donations are invaluable in many critical treatments, and initiatives like AXA Mansard’s blood drive bring immense relief to healthcare system.
“We are sincerely grateful for this partnership and the dedication of AXA Mansard’s employees,” Fakeye stated.
AXA Hearts in Action operates globally, she urging AXA employees everywhere to engage with and give back to their communities.
Through diverse projects – from health initiatives like this blood drive and medical outreaches to environmental efforts like the AXA Week for Good “Trash-to-Treasure” waste management project – AXA staff have opportunities to make a lasting difference on issues that matter.
For AXA Mansard, every act of social service brings them closer to the communities they serve, helping build a world where giving back is not only about charity but about lasting, positive change.
-

 Feature/OPED5 years ago
Feature/OPED5 years agoDavos was Different this year
-
Travel/Tourism8 years ago
Lagos Seals Western Lodge Hotel In Ikorodu
-

 Showbiz2 years ago
Showbiz2 years agoEstranged Lover Releases Videos of Empress Njamah Bathing
-

 Banking6 years ago
Banking6 years agoSort Codes of GTBank Branches in Nigeria
-

 Economy2 years ago
Economy2 years agoSubsidy Removal: CNG at N130 Per Litre Cheaper Than Petrol—IPMAN
-

 Banking2 years ago
Banking2 years agoFirst Bank Announces Planned Downtime
-

 Sports2 years ago
Sports2 years agoHighest Paid Nigerian Footballer – How Much Do Nigerian Footballers Earn
-

 Technology4 years ago
Technology4 years agoHow To Link Your MTN, Airtel, Glo, 9mobile Lines to NIN


















